ICH E5(R1) 接受国外临床试验数据的种族因素
1. 前言
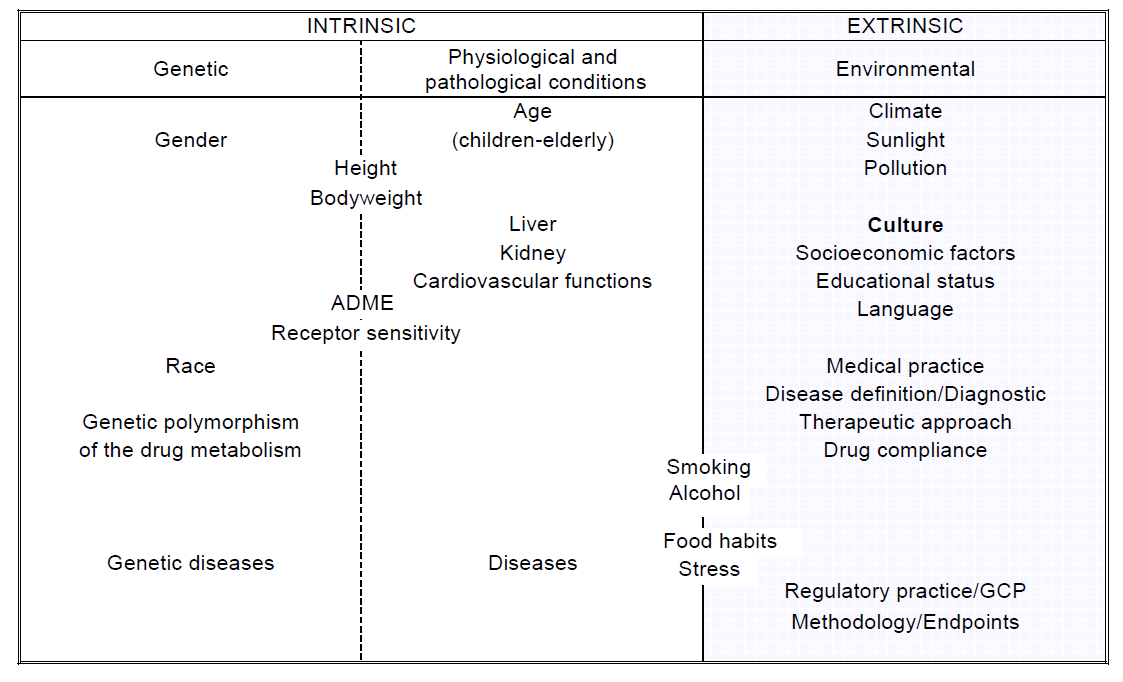
本指南的目的,是推荐一个用于评估种族因素对药物疗效的影响的框架,即某一特定剂量和给药方案对该药的安全性和有效性的影响,从而帮助药品在国际协调会议(ICH)地区注册。本指南为监管和研发策略提供指导,尽可能减少重复临床研究,尽快为患者提供药物使其获益的同时,又对种族因素的影响进行了充分的评估。本指南将与其他ICH 指南一起实施。根据本文件的目的,种族因素被定义为人群中与遗传和生理因素(内因)、以及文化和环境(外因)特征有关的因素。(附录A).
1.1 Objectives目的
To describe the characteristics of foreign clinical data that will facilitate their extrapolation to different populations and support their acceptance as a basis for registration of a medicine in a new region*.描述国外临床试验数据的特征,以便将其外推到不同人群,支持药品在新地区注册。To describe regulatory strategies that minimize duplication of clinical data and facilitate acceptance of foreign clinical data in the new region.尽量减少重复的临床研究,及促进新地区接受国外临床试验数据的监管策略。To describe the use of bridging studies*, when necessary, to allow extrapolation of foreign clinical data to a new region.应用桥接研究,必要时允许将国外临床试验数据外推到新地区。To describe development strategies capable of characterizing ethnic factor influences on safety, efficacy, dosage and dose regimen.能够表征种族因素对安全性、有效性、剂量和给药方案的影响的研发策略。
1.2 Background背景
All regions acknowledge the desirability of utilizing foreign clinical data that meet the regulatory standards and clinical trial practices acceptable to the region considering the application for registration.申请注册时,所有地区都接受符合法规标准及该地区申请注册要求的国外临床试验数据。
However, concern that ethnic differences may affect the medication’s safety, efficacy, dosage and dose regimen in the new region has limited the willingness to rely on foreign clinical data. Historically, this has been one of the reasons, therefore, the regulatory authority in the new region has often requested that all, or much of, the foreign clinical data in support of registration be duplicated in the new region. Although ethnic differences among populations may cause differences in a medicine’s safety, efficacy, dosage or dose regimen, many medicines have comparable characteristics and effects across regions. Requirements for extensive duplication of clinical evaluation for every compound can delay the availability of new therapies and unnecessarily waste drug development resources.但是种族差异可能影响药物的安全性、有效性、剂量和给药方案,使得新地区对国外临床试验数据的接受程度受到限制。这也是以往在新地区提交注册申请时,监管机构要求其在新地区完全或大部分重复国外临床研究和验证的原因之一。虽然不同人群之间的种族差异可能导致药物的安全性、疗效、剂量或给药方案的差异,但很多药物在不同地区的人群之间具有相似的特征和作用。要求对每一个药物进行大量重复的临床研究,可能会延迟新疗法的应用和浪费不必要的药物研发资源。
1.3 Scope范围
This guidance is based on the premise that it is not necessary to repeat the entire clinical drug development program in the new region and is intended to recommend strategies for accepting foreign clinical data as full or partial support for approval of an application in a new region. It is critical to appreciate that this guidance is not intended to alter the data requirements for registration in the new region; it seeks to recommend when these data requirements may be satisfied with foreign clinical data. All data in the clinical data package, including foreign data, should meet the standards of the new region with respect to study design and conduct and the available data should satisfy the regulatory requirements in the new region. Additional studies conducted in any region may be required by the new region to complete the clinical data package.本指南的前提是,没有必要在新地区重复进行药物的全部临床研发过程,建议全部或部分的接受国外临床研究数据,以支持药物在新地区的注册审批。首先需要申明的是,本指南并不是为了在新地区申请注册药品而修改对临床数据的要求,而是旨在国外临床研究数据可能符合新地区的注册要求时,推荐接受国外临床资料。临床数据集的所有数据资料,包括国外数据,必须符合新地区的研究设计和实施标准,遵循新地区的监管要求。新地区可要求申办者在该地区进行附加研究以完善临床数据集。
Once a clinical data package fulfils the regulatory requirements of the new region, the only remaining issue with respect to the acceptance of the foreign clinical data is its ability to be extrapolated to the population of the new region. When the regulatory authority or the sponsor is concerned that differences in ethnic factors could alter the efficacy or safety of the medicine in the population in the new region, the sponsor may need to generate a limited amount of clinical data in the new region in order to extrapolate or “bridge” the clinical data between the two regions.若现有临床数据集符合新地区的管理要求,这些国外数据是否能被接受,还取决于该数据能否外推到新地区的人群。当监管机构或申办者认为种族因素可能改变药物在新地区人群中的安全性或有效性时,申办者可能需要在新地区获得一定的临床数据,以便将两个地区之间的临床数据外推或桥接起来。
If a sponsor needs to obtain additional clinical data to fulfil the regulatory requirements of the new region, it is possible that these clinical trials can be designed to also serve as the 如果申办者需要获得额外的临床数据,以满足新地区的监管要求,则可以将这些临床试验设计成桥接研究(bridging studies.study)。
Thus, the sponsor and the regional regulatory authority of the new region would assess an application for registration for:因此,申办者和新地区的监管机构对于该项注册申请需要评估的内容包括:
Thus, the sponsor and the regional regulatory authority of the new region would assess an application for registration for:(1)完全符合新地区监管要求;
its completeness with respect to the regulatory requirements of the new region; and
the ability to extrapolate to the new region those parts of the application (which could be most or all of the application) based on studies from the foreign region ((2)将国外临床研究中的部分(大部分或全部)数据应用到新地区的可能性。(Appendix B附录B).)
2. ASSESSMENT OF THE CLINICAL DATA PACKAGE INCLUDING FOREIGN CLINICAL DATA FOR ITS FULFILMENT OF REGULATORY REQUIREMENTS IN THE NEW REGION评估包括国外临床数据的临床数据集,以满足新地区的监管要求
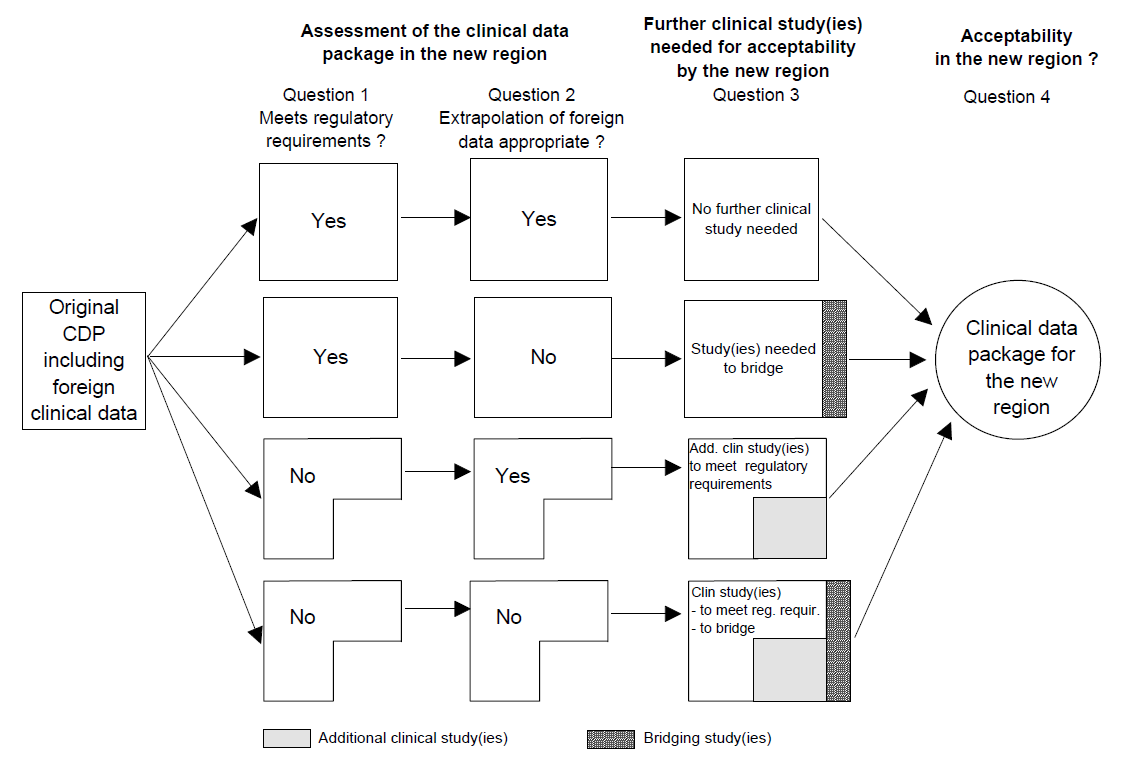
The regional regulatory authority would assess the clinical data package, including the foreign data, as to whether or not it meets all of the regulatory standards regarding the nature and quality of the data, irrespective of its geographic origin, i.e., data generated either totally in a foreign region (or regions) or data from studies conducted both in a foreign and the new region to which the application is being made. A clinical data package that meets all of these regional regulatory requirements is defined as a “Complete” Clinical Data Package* for submission and potential approval. The acceptability of the foreign clinical data component of the complete data package depends then upon whether it can be extrapolated to the population of the new region.新地区的监管机构将评估包括国外临床数据的临床数据集,以确定该数据的性质和质量是否符合该地区所有的监管标准,而不考虑该数据全部来源于国外、部分来源于国外、还是部分来源于即将申请注册的地区。可通过审批的完整临床数据集的定义为,符合所有地区监管要求的临床数据集。完整临床数据集中的国外临床数据是否被接受,取决于它能否外推到新地区人群中。
Before extrapolation can be considered, the Complete Clinical Data Package, including foreign clinical data, submitted to the new region should contain:在考虑外推之前,递交给新地区的包括国外临床数据的完整临床数据集应当包括以下内容:
Adequate characterization of pharmacokinetics*, pharmacodynamics*, dose-response, efficacy and safety in the population of the foreign region(s).国外人群的药动学、药效学、量-效关系、安全性和有效性特点;Clinical trials establishing dose response, efficacy and safety. These trials should:确立药物量-效关系、有效性和安全性的临床研究。这些研究包括:Be designed and conducted according to regulatory standards in the new region, e.g., choice of controls, and should be conducted according to GCP根据新地区监管标准进行设计和实施,例如对照药的选择,且遵照临床试验管理规范(GCP)实施;Be adequate and well-controlled*研究充分,且具有良好的对照;Utilize endpoints that are considered appropriate for assessment of treatment采用合适的治疗终点进行评价;Evaluate clinical disorders using medical and diagnostic definitions that are acceptable to the new region.疾病评估时所采用的治疗和诊断的定义能够被新地区所接受。Characterization in a population relevant to the new region of the pharmacokinetics, and where possible, pharmacodynamics and dose response for pharmacodynamic endpoints. This characterization could be performed in the foreign region in a population representative of the new region* or in the new region*.描述新地区人群的药动学特征,以及(在可能的情况下)药效学特征和以药效学为终点指标的量-效关系特征。应在能够代表新地区人群的国外人群,或在新地区人群中开展研究。
Several针对临床试验的设计、实施、分析和报告各方面的一系列ICH ICH指南,将有助于实现完整临床数据集的概念。这些指南包括 guidelines that address aspects of design, conduct, analysis and reporting of clinical trials will help implement the concepts of the Complete Clinical Data Package. These guidances include GCP’s (E6), evaluation of dose response (E4), adequacy of safety data (GCP(E6)、量-效关系评估(E4)、充分的安全性数据(E1 and E2), conduct of studies in the elderly (E7), reporting of study results (E3), general considerations for clinical trials (E8), and statistical considerations (E9). A guidance on the choice of control group in clinical trials (E10) is under development.和E2)、老年用药研究(E7)、研究结果的报告(E3)、一般临床试验的总体考虑(E8)及统计学考虑(E9)。临床试验对照组的选择指导原则(E10)尚待完善。
2.1 Additional Studies to Meet the New Region’s Regulatory Requirements根据新地区监管要求的附加临床研究
When the foreign clinical data do not meet the regional regulatory requirements, the regulatory authority may require additional clinical trials such as:当国外临床资料不符合新地区的监管要求时,新地区的监管机构可能会要求增加临床试验,例如:
clinical trials in different subsets of the population such as patients with renal insufficiency, patients with hepatic dysfunction, etc.增加在特殊人群中的临床试验:如肾功能不全及肝功能不全的病人等;clinical trials using different comparators at the new region’s approved dosage and dose regimen按照新地区批准的剂量和给药方法,以不同的对照药进行临床试验;drug-drug interaction studies药物相互作用研究。
3. ASSESSMENT OF THE FOREIGN CLINICAL DATA FOR EXTRAPOLATION TO THE NEW REGION评估国外临床试验数据从而外推到新地区
3.1 Characterization of the Medicine’s Sensitivity to Ethnic Factors药物的种族敏感性特征
knowledge评估一个药物对种族因素的敏感性,必须了解它的药动学和药效学特征以及应用这些特征解释临床安全性和有效性。附录 ofC its pharmacokinetic and pharmacodynamic properties and the translation of those properties to clinical effectiveness and safety. A reasonable evaluation is described in Appendix C. Some properties of a medicine (chemical class, metabolic pathway, pharmacologic class) make it more or less likely to be affected by ethnic factors (Appendix D). Characterization of a medicine as “ethnically insensitive”, i.e., unlikely to behave differently in different populations, would usually make it easier to extrapolate data from one region to another and need less bridging data.中描述了一种合理的评估方法。药物的某些特性(化学分类,代谢途径,药理学分类)决定了该药物更容易或不易受到种族因素的影响(附录D)。药物对种族因素不敏感,即不太可能在不同人群中表现出差异,通常会使国外临床数据更容易由一个地区外推到另一个地区中,并且需要较少的桥接数据。
Factors that make a medicine ethnically sensitive or insensitive will become better understood and documented as effects in different regions are compared. It is clear at present, however, that such characteristics as clearance by an enzyme showing genetic polymorphism and a steep dose-response curve will make ethnic differences more likely. Conversely, a lack of metabolism or active excretion, a wide therapeutic dose range*, and a flat dose response curve will make ethnic differences less likely. The clinical experience with other members of the drug class in the new region will also contribute to the assessment of the medicine’s sensitivity to ethnic factors. It may be easier to conclude that the pharmacodynamic and clinical behaviour of a medicine will be similar in the foreign and new regions if other members of the pharmacologic class have been studied and approved in the new region with dosing regimens similar to those used in the original region.通过比较不同地区的药物效应,药物对种族因素是否敏感将变得更容易理解和评价。然而,显而易见的是,若药物的代谢酶存在基因多态性、量-效曲线陡峭,将更可能存在种族差异。相反,药物缺乏代谢或主动排泄、治疗窗宽、以及量-效曲线平缓,则不易出现种族差异。同类药物在新地区应用的临床经验,也有助于评估药物对种族因素的敏感性。如果在新地区中,相同药理学特征的同类药物,采用与原地区相同的剂量和给药方案进行研究并获批,可能较容易得出药物的药效学和临床行为在国外和新地区也是类似的结论。
3.2 Bridging Data Package桥接数据集
3.2.1 Definition of Bridging Data Package and Bridging Study桥接数据集和桥接研究的定义
A bridging data package consists of: 1) selected information from the Complete Clinical Data Package that is relevant to the population of the new region, including pharmacokinetic data, and any preliminary pharmacodynamic and dose-response data, and 2) if needed, a bridging study to extrapolate the foreign efficacy data and/or safety data to the new region.一个桥接数据集包括:(1)从完整临床数据集中选出与新地区人群相关的信息,包括药动学数据,药效学和量-效关系数据;(2)有可能需要将国外的有效性和/或安全性数据外推到新地区的桥接研究数据。
A bridging study is defined as a study performed in the new region to provide pharmacodynamic or clinical data on efficacy, safety, dosage and dose regimen in the new region that will allow extrapolation of the foreign clinical data to the population in the new region. A bridging study for efficacy could provide additional pharmacokinetic information in the population of the new region. When no bridging study is needed to provide clinical data for efficacy, a pharmacokinetic study in the new region may be considered as a bridging study.桥接研究的定义是,在新地区进行的一项研究,旨在提供新地区的有效性、安全性、剂量和给药方案的药效学或临床数据,从而能够将国外临床数据外推到新地区人群。有效性的桥接研究可以为新地区人群提供额外的药动学信息。当不需要桥接研究提供临床疗效数据时,在新地区进行的药动学研究即可看做是桥接研究。
3.2.2 Nature and Extent of the Bridging Study桥接研究的类型与范围
This guidance proposes that when the regulatory authority of the new region is presented with a clinical data package that fulfils its regulatory requirements, the authority should request only those additional data necessary to assess the ability to extrapolate foreign data from the Complete Clinical Data Package to the new region. The sensitivity of the medicine to ethnic factors will help determine the amount of such data. In most cases, a single trial that successfully provides these data in the new region and confirms the ability to extrapolate data from the original region should suffice and should not need further replication. Note that even though a single study should be sufficient to “bridge” efficacy data, a sponsor may find it practical to obtain the necessary data by conducting more than one study. For example, where it is intended that a fixed dose, dose-response study using a clinical endpoint is needed as the bridging study, a short-term pharmacologic endpoint study may be used to choose the dose(s) for the larger (clinical endpoint) study.本指南建议,当新地区监管机构收到符合其监管要求的临床数据集时,应要求递交完整临床数据集中可外推到新地区的必须附加的数据资料集。药物是否存在种族敏感性,决定了这些数据的数量。多数情况下,在新地区进行一个单独的临床试验就能提供这些数据,以满足由原地区外推到新地区的需要,不必再进一步开展重复研究。值得注意的是,虽然有时一个桥接研究就足够用来桥接药物的有效性数据,但实际上申办者可能需要开展更多的研究以获得必要的资料。例如,如果需要采用临床终点,固定剂量的量-效关系研究作为桥接研究,可以用短期的临床药理学终点研究来为较大规模(临床终点)的研究选择给药剂量。
When the regulatory authority requests, or the sponsor decides to conduct, a bridging study, discussion between the regional regulatory authority and sponsor is encouraged, when possible, to determine what kind of bridging study will be needed. The relative ethnic sensitivity will help determine the need for and the nature of the bridging study. For regions with little experience with registration based on foreign clinical data, the regulatory authorities may still request a bridging study for approval even for compounds insensitive to ethnic factors. As experience with interregional acceptance increases, there will be a better understanding of situations in which bridging studies are needed. It is hoped that with experience, the need for bridging data will lessen.当监管机构要求,或申办者决定实施一个桥接研究,在可能的情况下,鼓励他们通过讨论决定实施何种类型的桥接研究。相对的种族敏感性有助于决定是否进行桥接研究以及进行何种类型的桥接研究。对于没有使用国外数据进行药品注册的经验的地区,即使该药物无种族敏感性,监管机构仍应要求一个桥接研究。当地区间互相接受数据的经验增加,对桥接研究的必要性会有更好的认识。希望随着经验丰富,减少对桥接研究数据的需求。
The following is general guidance about the ability to extrapolate data generated from a bridging study:基于桥接研究数据进行外推的总体指南如下:
If the bridging study shows that dose response, safety and efficacy in the new region are similar, then the study is readily interpreted as capable of “bridging” the foreign data.如果桥接研究证实在新地区的量-效关系、安全性和有效性与国外相似,则该研究即可说明其能够桥接国外数据。If如果一个实施恰当的桥接研究表明,在新地区不同剂量下的安全性和有效性结果与原地区没有较大差异,通常可将国外数据外推到新a bridging study, properly executed, indicates that a different dose in the new region results in a safety and efficacy profile that is not substantially different from that derived in the original region, it will often be possible to extrapolate the foreign data to the new region, with appropriate dose adjustment, if this can be adequately justified (e.g., by pharmacokinetic and/or pharmacodynamic data).地区,也可通过适当的剂量调整(采用药动学和/或药效学数据)将国外数据外推到新地区。If the bridging study designed to extrapolate the foreign data is not of sufficient size to confirm adequately the extrapolation of the adverse event profile to the new population, additional safety data may be necessary (section如果桥接研究的规模不能充分描述新地区的不良反应情况,从而将国外数据外推至新地区,则必须增加安全性数据(3.2.4).4 部分)。If the bridging study fails to verify safety and efficacy, additional clinical data (e.g., confirmatory clinical trials) would be necessary.如果桥接研究未能验证药物的安全性和有效性,则需要额外的临床数据(如确证性临床研究)。
3.2.3 Bridging Studies for Efficacy有效性的桥接研究
Generally, for medicines characterized as insensitive to ethnic factors, the type of bridging study needed (if needed) will depend upon experience with the drug class and upon the likelihood that extrinsic ethnic factors (including design and conduct of clinical trials) could affect the medicine’s safety, efficacy, and dose-response. For medicines that are ethnically sensitive, a bridging study may often be needed if the populations in the two regions are different. The following examples illustrate types of bridging studies for consideration in different situations:通常对种族因素不敏感的药物,所需桥接研究(如果需要)的类型取决于此类药物的用药经验和外在的种族因素(包括临床试验的设计与实施)对药物安全性、有效性以及量-效关系可能存在的影响。对于种族因素敏感的药物,如果两个地区人群有差异,通常需要桥接研究。以下示例说明了不同情况下应考虑采用的桥接研究类型:
No Bridging Study无桥接研究
In some situations, extrapolation of clinical data may be feasible without a bridging study:如果药物对种族不敏感,并且外在因素如医疗措施和临床试验的实施在两地区大致相同。
If the medicine is ethnically insensitive and extrinsic factors such as medical practice and conduct of clinical trials in the two regions are generally similar.
If the medicine is ethnically sensitive but the two regions are ethnically similar and there is sufficient clinical experience with pharmacologically related compounds to provide reassurance that the class behaves similarly in patients in the two regions with respect to efficacy, safety, dosage and dose regimen. This might be the case for well-established classes of drugs known to be administered similarly but not necessarily identically in the two regions.如果药物对种族敏感,但两地区种族相似,并且药理机制类似的药物有足够的临床经验,可保证该类药物在两地区病人中的安全性、有效性、剂量和给药方案方面相似。这可能是对于给药方式类似的同类药物的情况,同类药物在两个地区应用情况类似,但不一定相同。
Bridging Studies using pharmacologic endpoints临床药理学终点的桥接研究
If the regions are ethnically dissimilar and the medicine is ethnically sensitive but extrinsic factors are generally similar (e.g., medical practice, design and conduct of clinical trials) and the drug class is a familiar one in the new region, a controlled pharmacodynamic study in the new region, using a pharmacologic endpoint that is thought to reflect relevant drug activity (which could be a well-established surrogate endpoint) could provide assurance that the efficacy, safety, dose and dose regimen data developed in the first region are applicable to the new region. Simultaneous pharmacokinetic (i.e., blood concentration) measurements may make such studies more interpretable.如果两地区之间有种族差异,而且药物对种族敏感,但外在因素大致相同(如医疗实践,临床试验设计和实施),且该类药物在新地区有临床经验,则在新地区采用反映药物活性的药理学终点(经过验证的替代终点)进行对照的药效学研究,可保证在原地区建立的有效性、安全性、剂量和给药方案适用于新地区。同时药动学(即血药浓度)监测可使这些研究更有说服力。
Controlled Clinical Trials对照临床试验
It will usually be necessary to carry out a controlled clinical trial, often a randomized, fixed dose, dose-response study, in the new region when:在以下情况,通常需要在新地区进行对照试验,常为随机、固定剂量的量-效关系研究:
1. there are doubts about the choice of dose,对剂量的选择有疑问时;
2. there is little or no experience with acceptance of controlled clinical trials carried out in the foreign region,缺乏接受国外对照临床试验数据的经验;
3. medical practice, e.g., use of concomitant medications and design and/or conduct of clinical trials are different, or医学实践不同如合并用药不同,临床试验的设计和/或实施不同;
4. the drug class is not a familiar one in the new region.新地区对此类药物不熟悉。
Depending on the situation, the trial could replicate the foreign study or could utilize a standard clinical endpoint in a study of shorter duration than the foreign studies or utilize a validated surrogate endpoint, e.g., blood pressure or cholesterol (longer studies and other endpoints may have been used in the foreign phase III clinical trials).根据这些具体情况,可以重复国外临床研究,或采用标准的临床终点进行短期研究,或采用经过验证的替代终点,如血压或胆固醇(国外三期临床试验中可能已采用更长时间的研究和其他终点)。
If pharmacodynamic data suggest that there are interregional differences in response, it will generally be necessary to carry out a controlled trial with clinical endpoints in the new region. Pharmacokinetic differences may not always create that necessity, as dosage adjustments in some cases might be made without new trials. However, any substantial difference in metabolic pattern may often indicate a need for a controlled clinical trial.如果药效学数据提示地区间疗效有差异,通常有必要在新地区进行一项临床终点的对照试验。药动学的差异并不一定需要进行这样的对照试验,因为在某些情况下,只需调整剂量而不需要进行新的临床试验,但代谢方式存在本质区别时,通常提示需要进行对照临床试验。
When the practice of medicine differs significantly in the use of concomitant medications, or adjunct therapy could alter the medicine’s efficacy or safety, the bridging study should be a controlled clinical trial.当医学实践在合并用药方面存在显著差异,或者辅助治疗可能改变药物的安全性或有效性时,那么桥接研究应为一项对照临床试验。
3.2.4 Bridging Studies for Safety安全性的桥接研究
Even即使国外临床数据已表明药物在国外应用的有效性和安全性,有时在新地区仍可能出现需要关注的安全性问题,这包括对新地区常见不良事件发生率的精确估计,以及严重事件的发现(1%的发生率通常需要评估约300 though the foreign clinical data demonstrate efficacy and safety in the foreign region, there may occasionally remain a safety concern in the new region. Safety concerns could include the accurate determination of the rates of relatively common adverse events in the new region and the detection of serious adverse events (in the 1% range and generally needing about 300 patients to assess). Depending upon the nature of the safety concern, safety data could be obtained in the following situations:例患者)。依据安全性问题的性质,在下述情况下需要获得安全性资料:
A评估有效性的桥接研究(例如量-效关系研究),因能估计常见的不良反应发生率,也可识别新地区更常见的严重不良事件,从而更具bridging study to assess efficacy, such as a dose-response study, could be powered to address the rates of common adverse events and could also allow identification of serious adverse events that occur more commonly in the new region. Close monitoring of such a trial would allow recognition of such serious events before an unnecessarily large number of patients in the new region is exposed. Alternatively, a small safety study could precede the bridging study to provide assurance that serious adverse effects were not occurring at a high rate.有说服力。在新地区对这样的试验进行密切监测,将有助于识别这类严重事件,避免药物暴露到新地区的大量患者当中。或者,可以在桥接研究之前进行一项小规模的安全性研究,以确保严重不良反应不会高频率发生。If there is no efficacy bridging study needed or if the efficacy bridging study is too small or of insufficient duration to provide adequate safety information, a separate safety study may be needed. This could occur where there is:如果不需要有效性桥接研究,或有效性桥接研究规模过小,或研究时间过短不足以提供充分的安全性信息,则可能需要进行单独的安全性研究,可见于以下情况:an index case of a serious adverse event in the foreign clinical data国外临床数据中有严重不良反应的病例;a concern about differences in reporting adverse events in the foreign region新地区与国外报道的不良反应存在差异的;only limited safety data in the new region arising from an efficacy bridging study, inadequate to extrapolate important aspects of the safety profile, such as rates of common adverse events or of more serious adverse events新地区只有有限的来源于药效桥接研究的安全性数据,不足以外推到安全性的重要方面,例如,常见不良反应发生率或更严重的不良反应。
4. DEVELOPMENTAL STRATEGIES FOR GLOBAL DEVELOPMENT全球研发策略
Definition of not only pharmacokinetics but also pharmacodynamics and dose response early in the development program may facilitate the determination of the need for, and nature of, any requisite bridging data. Any candidate medicine for global development should be characterized as ethnically sensitive or insensitive (Appendix D). Ideally, this characterization should be conducted during the early clinical phases of drug development, i.e., human pharmacology and therapeutic exploratory studies. In some cases, it may be useful to discuss bridging study designs with regulatory agencies prior to completion of the clinical data package. However, analysis of the data within the Complete Clinical Data Package will determine the need for, and type of bridging study. For global development, studies should include populations representative of the regions where the medicine is to be registered and should be conducted according to ICH guidelines.
A sponsor may wish to leave the assessment of pharmacokinetics, pharmacodynamics, dosage and dose regimens in populations relevant to the new region until later in the drug development program. Pharmacokinetic assessment could be accomplished by formal pharmacokinetic studies or by applying population pharmacokinetic methods to clinical trials conducted either in a population relevant to the new region, or in the new region.
5. SUMMARY
This guidance describes how a sponsor developing a medicine for a new region can deal with the possibility that ethnic factors could influence the effects (safety and efficacy) of medicines and the risk/benefit assessment in different populations. Results from the foreign clinical trials could comprise most, or in some cases, all of the clinical data package for approval in the new region, so long as they are carried out according to the requirements of the new region. Acceptance in the new region of such foreign clinical data may be achieved by generating “bridging” data in order to extrapolate the safety and efficacy data from the population in the foreign region(s) to the population in the new region.
GLOSSARY
| Term | Content |
|---|---|
| Adequate and Well-controlled Trial |
An adequate and well controlled trial has the following characteristics:
|
| Bridging Data Package | Selected information from the Complete Clinical Data Package that is relevant to the population of the new region, including pharmacokinetic data, and any preliminary pharmacodynamic and dose-response data and, if needed, supplemental data obtained from a bridging study in the new region that will allow extrapolation of the foreign safety and efficacy data to the population of the new region. |
| Bridging Study | A bridging study is defined as a supplemental study performed in the new region to provide pharmacodynamic or clinical data on efficacy, safety, dosage and dose regimen in the new region that will allow extrapolation of the foreign clinical data to the new region. Such studies could include additional pharmacokinetic information. |
| Complete Clinical Data Package | A clinical data package intended for registration containing clinical data that fulfil the regulatory requirements of the new region and containing pharmacokinetic data relevant to the population in the new region. |
| Compounds Insensitive to Ethnic Factors | A compound whose characteristics suggest minimal potential for clinically significant impact by ethnic factors on safety, efficacy, or dose response. |
| Compounds Sensitive to Ethnic Factors | A compound whose pharmacokinetic, pharmacodynamic, or other characteristics suggest the potential for clinically significant impact by intrinsic and/or extrinsic ethnic factors on safety, efficacy, or dose response. |
| Dosage | The quantity of a medicine given per administration, or per day. |
| Dose Regimen | The route, frequency and duration of administration of the dose of a medicine over a period of time. |
| Ethnic Factors |
The word ethnicity is derived from the Greek word “ethnos”, meaning nation or people. Ethnic factors are factors relating to races or large populations grouped according to common traits and customs. Note that this definition gives ethnicity, by virtue of its cultural as well as genetic implications, a broader meaning than racial. Ethnic factors may be classified as either intrinsic or extrinsic. (Appendix A)
|
| Extrapolation of Foreign Clinical Data | The generalization and application of the safety, efficacy and dose response data generated in a population of a foreign region to the population of the new region. |
| Foreign Clinical Data | Foreign clinical data is defined as clinical data generated outside of the new region (i.e., in the foreign region). |
| ICH Regions | European Union, Japan, The United States of America. |
| New Region | The region where product registration is sought. |
| Population Representative of the New Region | A population that includes the major racial groups within the new region. |
| Pharmacokinetic Study | A study of how a medicine is handled by the body, usually involving measurement of blood concentrations of drug and its metabolite(s) (sometimes concentrations in urine or tissues) as a function of time. Pharmacokinetic studies are used to characterize absorption, distribution, metabolism and excretion of a drug, either in blood or in other pertinent locations. When combined with pharmacodynamic measures (a PK/PD study) it can characterize the relation of blood concentrations to the extent and timing of pharmacodynamic effects. |
| Pharmacodynamic Study | A study of a pharmacological or clinical effect of the medicine in individuals to describe the relation of the effect to dose or drug concentration. A pharmacodynamic effect can be a potentially adverse effect (anticholinergic effect with a tricyclic), a measure of activity thought related to clinical benefit (various measures of beta-blockade, effect on ECG intervals, inhibition of ACE or of angiotensin I or II response), a short term desired effect, often a surrogate endpoint (blood pressure, cholesterol), or the ultimate intended clinical benefit (effects on pain, depression, sudden death). |
| Population Pharmacokinetic Methods | Population pharmacokinetic methods are a population-based evaluation of measurements of systemic drug concentrations, usually two or more per patient under steady state conditions, from all, or a defined subset of, patients who participate in clinical trials. |
| Therapeutic Dose Range | The difference between the lowest effective dose and the highest dose that gives further benefit. |
APPENDIX A
Classification of intrinsic and extrinsic ethnic factors
APPENDIX B
Assessment of the clinical data package (CDP) for acceptability
APPENDIX C
Pharmacokinetic, Pharmacodynamic, and Dose Response Considerations
Evaluation of the pharmacokinetics and pharmacodynamics, and their comparability, in the three major racial groups most relevant to the ICH regions (Asian, Black, and Caucasian) is critical to the registration of medicines in the ICH regions. Basic pharmacokinetic evaluation should characterize absorption, distribution, metabolism, excretion (ADME), and where appropriate, food-drug and drug-drug interactions.
Adequate pharmacokinetic comparison between populations of the two regions allows rational consideration of what kinds of further pharmacodynamic and clinical studies (bridging studies) are needed in the new region. In contrast to the pharmacokinetics of a medication, where differences between populations may be attributed primarily to intrinsic ethnic factors and are readily identified, the pharmacodynamic response (clinical effectiveness, safety, and dose-response) may be influenced by both intrinsic and extrinsic ethnic factors and this may be difficult to identify except by conducting clinical studies in the new region.
The ICH-E4 document describes various approaches to dose-response evaluation. In general, dose-response (or concentration response) should be evaluated for both pharmacologic effect (where one is considered pertinent) and clinical endpoints in the foreign region. The pharmacologic effect, including dose-response, may also be evaluated in the foreign region in a population representative of the new region. Depending on the situation, data on clinical efficacy and dose-response in the new region may or may not be needed, e.g., if the drug class is familiar and the pharmacologic effect is closely linked to clinical effectiveness and dose-response, these foreign pharmacodynamic data may be a sufficient basis for approval and clinical endpoint and dose-response data may not be needed in the new region. The pharmacodynamic evaluation, and possible clinical evaluation (including dose-response) is important because of the possibility that the response curve may be shifted in a new population. Examples of this are well-documented, e.g., the decreased response in blood pressure of blacks to angiotensin-converting enzyme inhibitors.
APPENDIX D
A Medicine’s Sensitivity to Ethnic Factors
Characterization of a medicine according to the potential impact of ethnic factors upon its pharmacokinetics, pharmacodynamics and therapeutic effects may be useful in determining what sort of bridging study is needed in the new region. The impact of ethnic factors upon a medicine’s effect will vary depending upon the drug’s pharmacologic class and indication and the age and gender of the patient. No one property of the medicine is predictive of the compound’s relative sensitivity to ethnic factors. The type of bridging study needed is ultimately a matter of judgement but assessment of sensitivity to ethnic factors may help in that judgement.
The following properties of a compound make it less likely to be sensitive to ethnic factors:
- Linear pharmacokinetics (pK)
- A flat pharmacodynamic (PD) (effect-concentration) curve for both efficacy and safety in the range of the recommended dosage and dose regimen (this may mean that the medicine is well-tolerated)
- A wide therapeutic dose range* (again, possibly an indicator of good tolerability)
- Minimal metabolism or metabolism distributed among multiple pathways
- High bioavailability, thus less susceptibility to dietary absorption effects
- Low potential for protein binding
- Little potential for drug-drug, drug-diet and drug-disease interactions
- Non-systemic mode of action
- Little potential for inappropriate use
The following properties of a compound make it more likely to be sensitive to ethnic factors:
- Non-linear pharmacokinetics
- A steep pharmacodynamic curve for both efficacy and safety (a small change in dose results in a large change in effect) in the range of the recommended dosage and dose regimen
- A narrow therapeutic dose range
- Highly metabolized, especially through a single pathway, thereby increasing the potential for drug-drug interaction
- Metabolism by enzymes known to show genetic polymorphism
- Administration as a prodrug, with the potential for ethnically variable enzymatic conversion
- High inter-subject variation in bioavailability
- Low bioavailability, thus more susceptible to dietary absorption effects
- High likelihood of use in a setting of multiple co-medications
- High likelihood for inappropriate use , e.g., analgesics and tranquilizers.
